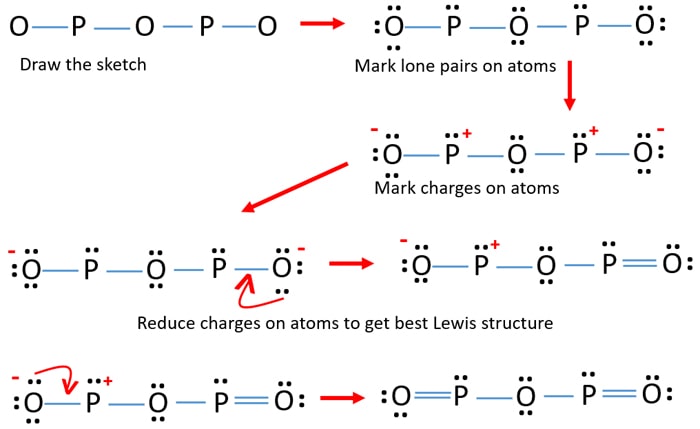
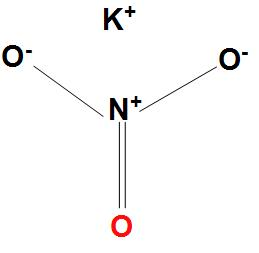
What is resonance? Are the resonance structures be the actual structure of molecules? Draw resonance structure of ozone (O) and Sulphur trioxide (SO).
Resonance is a way of describing delocalized electrons within certain molecules or polyatomic ions where the bonding cannot be expressed by a single Lewis formula. A molecule or ion with such delocalized electrons is represented by several resonance structures.
When it is possible to write more than one equivalent resonance structure for a molecule or ion, the actual structure is the average of the resonance structures. But resonance structures represent different ways of...








 -------->
-------->
 ------>
------>