1. Which of the following is correct expression of the first law of thermodynamics?a) q= ∆ H - Wb) ∆ H=q+Wc) ∆ U= q+ Wd) ∆ U= ∆ H +P ∆ V
2.The first law of thermodynamics fails to decide____________a) the direction ...
1. Which of the following is correct expression of the first law of thermodynamics?
a) q= ∆ H - W
b) ∆ H=q+W
c) ∆ U= q+ W
d) ∆ U= ∆ H +P ∆ V
2.The first law of thermodynamics fails to decide____________
a) the direction of the the process
b) the extent of conversion of one form of energy to another
c) both these
d) none of these
4.The first law of thermodynamics is also known as,
a) law of mass action
b) law of conservation of mass
c) law of conservation of energy
d) law of conservation of mass and energy
5. Heat cannot by itself flow from a body at a lower temperature to a body at a higher temperature”- the statement is which of the following?
First law of thermodynamics.
Conservation of mass.
Conservation of momentum.
Second law of thermodynamics.
6. Helium gas passes through a cycle ABCDA (containing two isobaric and isochoric lines) shown in the fig. Find the efficiency of the cycle. (Assume the gas to be close to ideal gas)
15.4%.
12.5%.
10.5%.
9.1%.
7. A Carnot engine, containing efficiency of ɳ=1/10 as heat engine and used as a refrigerator. The work done on the system is 10 J. Calculate the amount of energy absorbed at lower temperature from the reservoir.
1 J.
90 J.
99 J.
100 J.
8. Which statement is false?
Carnot cycle is reversible.
A reversible cycle is more efficient than irreversible one.
Carnot cycle is the most efficient among all cycles.
All reversible cycle has same efficiency.
9. The coefficient of performance of a refrigerator is 5. If the temperature inside said refrigerator is -20⁰ C. Calculate the temperature of the surrounding where it releases heat.
11⁰ C.
21⁰ C.
31⁰ C.
41⁰ C.
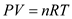 …… (1)
…… (1)
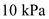 for P,
for P,  for V, 10 mol for n, and 8.31 for R.
for V, 10 mol for n, and 8.31 for R.
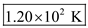 .
.