A cylinder containing 10.0 moles of mono atomic gas expands from A to B along the path shown in figure i) find the temperature of the gas at point A and the temperature at point B II)what is the ch...
From the ideal gas equation, the relation pressure P, volume V, and temperature of the gas can be expressed as,
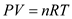 …… (1)
…… (1)
Here, n is number of moles.
From the above formula, the temperature of the gas at point A in the figure P 12.67 can be taken as,

Substitute 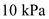 for P,
for P,  for V, 10 mol for n, and 8.31 for R.
for V, 10 mol for n, and 8.31 for R.

Therefore, the temperature of the gas at point A is 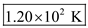 .
.
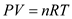 …… (1)
…… (1)
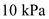 for P,
for P,  for V, 10 mol for n, and 8.31 for R.
for V, 10 mol for n, and 8.31 for R.
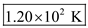 .
.