A steam engine intakes 200g of steam at 98 degree celsius per minute and cools it down to 10 degree celsius. Calculate the heat rejected by the steam engine per minute. Latent heat of vaporization ...
Heat rejected during condensation of steam per min = mL
= 200*540
...
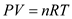 …… (1)
…… (1)
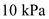 for P,
for P,  for V, 10 mol for n, and 8.31 for R.
for V, 10 mol for n, and 8.31 for R.
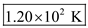 .
.