Faraday's Law of Electrolysis
Before moving into Faraday's Law of Electrolysis, let's talk about electrochemistry and some terms related to it. Electrochemistry The branch of chemistry that deals with the study of relation...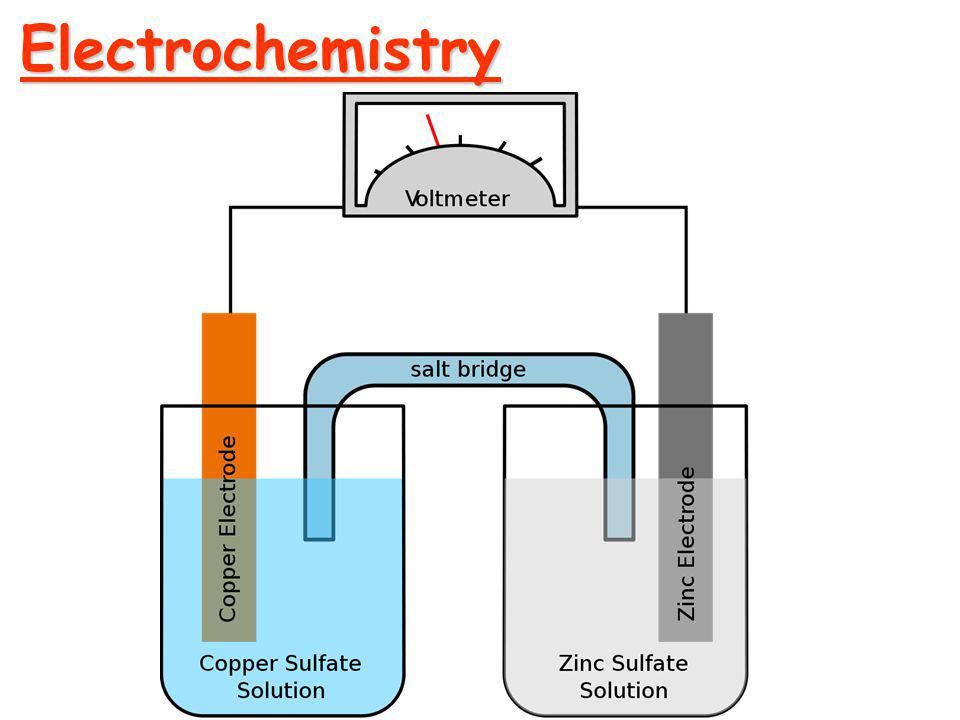

This law states that ” the mass of a substance deposited or liberated at any electrode on passing a certain amount of charge is directly proportional to its equivalent weight of the substance”.
That is w ∝ E
where w is the mass of the substance in grams while E is its chemical equivalent i.e. weight in gms per equivalent. This law can be explained as follows.
Consider three reactions, such as:
Na+ + e− –> Na
Cu2+ + 2e− –> Cu
Al3+ + 3e− –> Al
Assume that these three reactions are occurring in three separate electrolytic cells connected in series. When x moles of electrons are passed through the three cells, the mass of Na, Cu, and Al deposited are 23x gms, 31.75x gms, and 9x gms respectively. We can see that 23, 31.75 and 9 gm/eq are the chemical equivalent weights of the three elements.
∴ w = moles of electrons × E
The charge possessed by 1 mole of electrons = 1.6 × 10−19 × 6.023 x 1023 ≈ 96500 C
This charge is called as 1 Faraday.
If we pass one Faraday of charge, it means that we are passing one mole of electron and bypassing 1 Faraday of charge 1gm equivalent weight of the substance will be deposited or liberated.
∴ ![]()
![]()
(n = n-factor) By combining the first and second law, we get
![]()
Note: It should be made clear that the cathode is the electrode in which reduction reaction(s) occurs while the anode is the electrode where the oxidation reaction(s) occurs. Do not relate the sign (positive or negative) of the electrode with the nature of the electrode.