Electrochemical Series
- list of elements
- arranged in increasing order of their standard reduction potential values
- also known as activity series
Li K Ba Ca Na Mg Al Zn Cr Fe Sb Pb H2 Cu Fe Ag Au F2
Uses of Electrochemical Series
- To compare the oxidizing and reducing strengths.
Example:
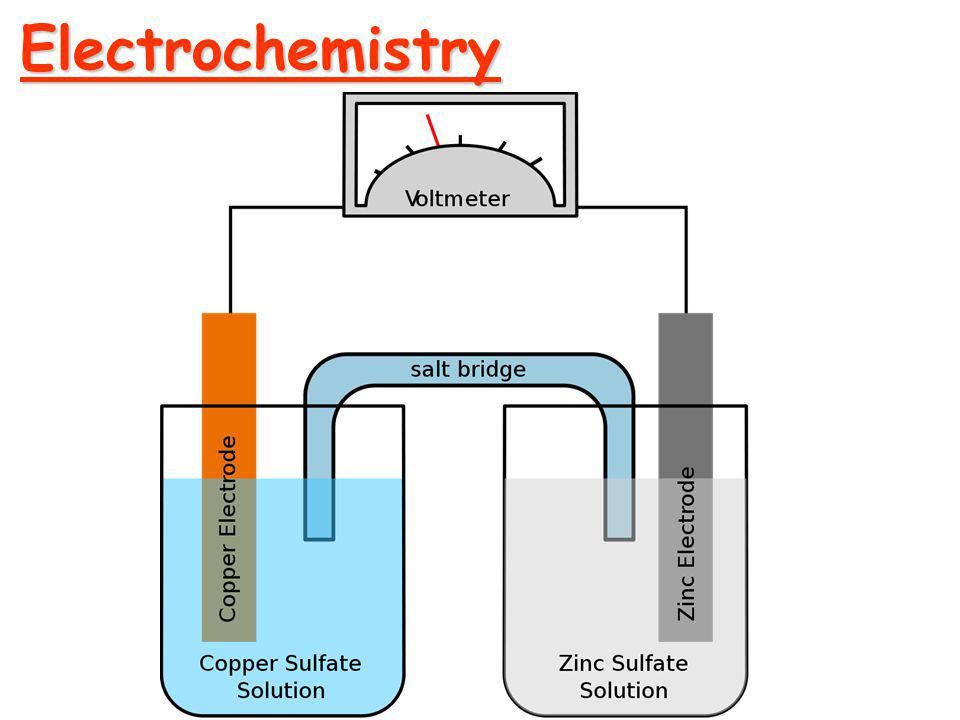
Cu++ + 2e- -----> Cu E0 = + 0.34
Zn++ + 2e- -----> Zn E0 = - 0.76
Since the E0 value of Cu++ is greater and can be easily reduced. Therefore, Cu++ is a stronger oxidizing agent the Zn++. In the electrochemical series, F2 is the strongest oxidizing agent and Li+ is the weakest oxidizing agent.
- Construction of Galvanic cell.
The electrode for a galvanic cell are chosen referencing the electrochemical series. Anode has lower reduction potential and cathode has higher reduction potential.
- Calculation of Emf of the cell.
The galvanic cell has two half cells: anode and cathode half cells.
Emf is the difference of the standard reduction potential of the cathode to anode.
Thus, E0(cell) = E0red(cathode) - E0red(anode)
- Prediction of the spontaneity of a reaction.
When emf of cell based on a specific redox reaction is calculated, if the emf is +ve, the reaction is spontaneous.
Zn/Zn++ // Cu++/Cu
E0(cell) = E0Zn/Zn++ - E0Cu++/Cu = 0.34V - ( - 0.76V ) = + 1.1V
So, the reaction is spontaneous.
- To predict whether the atom liberates hydrogen form acid or not.
The metal having a high tendency to lose e- can displace H+ ion form acid.
This means metal with low reduction potential can liberate hydrogen ion.
Li+ can liberate more H+ ion than Na+.
- To predict the relative reactivity of metals.
Reduction potential ↑ ------> tendency to loose elcetrons ↓ ------> reactivity ↓