Chemical Bonding and Shape of Molecules
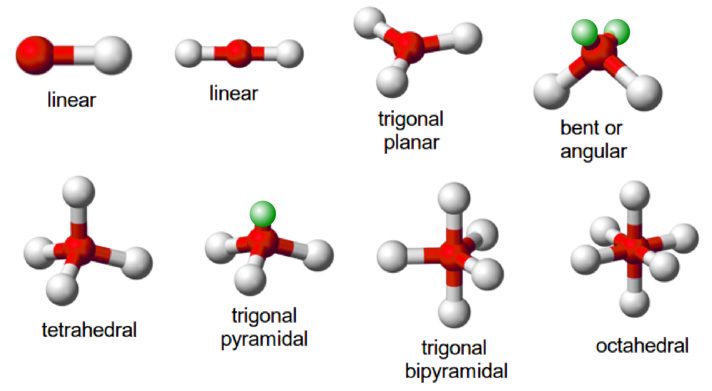

The Valence Shell Electron Pair Repulsion Theory abbreviated as VSEPR theory (proposed by Sedgwick and Powell 1940 and developed by Gillespie and Nyholm 1957 )is based on the premise that this arrangement of the atom determines the geometry of the resulting molecules there is a repulsion between the pairs of valence electrons in all atoms, and the atoms will always tend to arrange themselves in manner in which this electron pair repulsion is minimized.
In polyatomic molecules (i.e. molecules made up of three or more atoms), one of the constituent atoms is identified as the central atom to which all other atoms belonging to the molecule are linked.The total number of valence shell electron pairs decides the shape of the molecule. The electron pairs have a tendency to orient themselves in a way that minimizes the electron-electron repulsion between them and maximizes the distance between them. The valence shell can be thought of as a sphere where the electron pairs are localized in such a way that the distance between them is maximized.
The VSEPR theory can be applied to each resonance structure of a molecule. The strength of the repulsion is strongest in two lone pairs and weakest in two bond pairs. If electron pairs around the central atom are closer to each other, they will repel each other. This results in an increase in the energy of the molecules. If the electron pairs lie far from each other, the repulsions between them will be less and eventually, the energy of the molecule will be low.