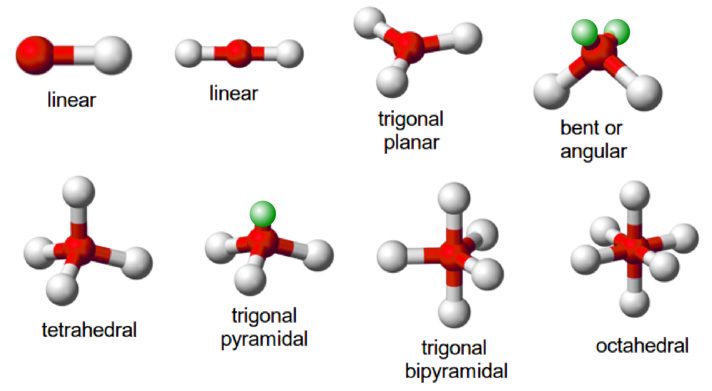
Chemical Bonding and Shape of Molecules

↪ Metals are characterized by bright, luster, high electrical and thermal conductivity, malleability, ductility and high tensile strength.
↪ A metallic crystal consists of a very large number of atoms arranged in a regular pattern.
↪ Different models have been proposed to explain the nature of metallic bonding. Two most important modules are as follows:

Electron Sea Model
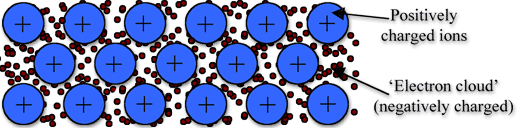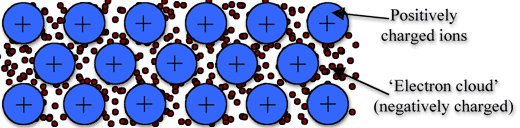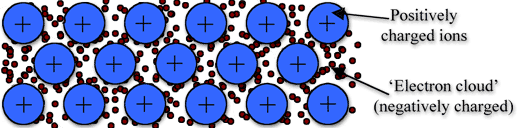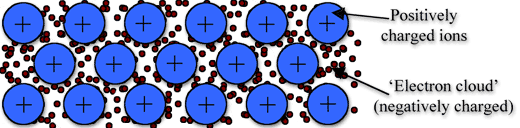
↪ In this model, a metal is assumed to consist of a lattice of positive ion (or kernels) immersed in a sea of mobile valence electrons, which move freely within the boundaries of a crystal.
↪ A positive kernel consists of the nucleus of the atom together with its core on a kernel is, therefore, equal in magnitude to the total valence electronic charge per atom.
↪ The free electrons shield the positively charged ion cores from mutual electrostatic repulsive forces which they would otherwise exert upon one another.
↪ In a way, these free electrons act as ‘glue’ to hold the ion cores together.
↪ The forces that hold the atoms together in a metal as a result of the attraction between positive ions and surrounding freely mobile electrons are known as metallic bonds.
Properties of metallic solids
1. Metallic luster:
↪ When light falls on the metal surface, free and mobile electrons get excited and jump into higher energy levels.
↪ While coming back into its ground state again, the excited electrons lose energy in the form of radiation.
↪ Therefore, the surface of metal emits a particular type of metallic luster.
2. Electrical conductivity:
↪ When an electrical field is applied in a metal, its free and mobile electrons act as charge carriers.
↪ Therefore, all metals conduct electricity
3. Malleability and ductility:
↪ When pressure is applied through a metal, new layers of the positively charged particles are formed instead of undergoing total deformation.
↪ Therefore, the metals are malleable and ductile.
4. Thermal conductivity:
↪ When heat is supplied through one part of the metal, the free electrons of that part gain kinetic energy.
↪ Since they are mobile, they move towards another part and transfer the kinetic energy they have gained.
↪ This means they transfer the heat quickly to another part of the metal.
5. Hardness:
↪ Metals are usually hard.
↪ This is due to the strong electrostatic force of attraction as all positively charged particles are surrounded by electrons.
6. High tensile strength:
↪ As kernel of metal ion is strongly bound by free electrons all around it, metals resist stretching without breaking the metal.
↪ This means, metals have high tensile strength.