Chemical Bonding and Shape of Molecules
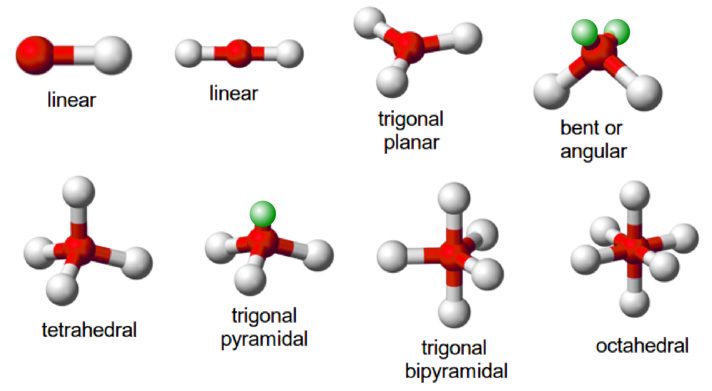

Definition.
Hybridization simply is the intermixing of atomic orbitals.
In other words, the process of mixing of orbitals of an atom of nearly equal energy giving rise to entirely new orbitals equal to in number to the mixing orbitals and having identical shape and same energy is called hybridization.
Characteristics.
These are the characteristics of hybridization.