Chemical Bonding and Shape of Molecules

↪ Valence bond theory (VBT) was proposed by Heitler and London in 1927 and improved by Linus Pauling to explain the nature of bonding in covalent molecules.
↪ This theory is based on wave mechanical model of atom.
↪ According to this theory, a covalent bond is formed by overlapping of atomic orbitals having unpaired electrons.
↪ The main postulates of this theory are as follows;
Postulates of VBT
1. Half-filled atomic orbital of one atom overlaps with half-filled atomic orbital of another atom to form a covalent bond.
2. Atomic orbitals undergoing overlap must be sufficiently close to each other with proper alignment.
3. The strength of bond formed depends upon the extent of overlapping of atomic orbitals. Greater the overlapping of atomic orbitals, stronger the covalent bond formed.
4. Overlapping lowers the energy of the molecule and excess energy is released. The energy released per mole is called stabilization energy or bond energy and the equilibrium distance between two nuclei of bonding atoms is bond length.
5. Number of unpaired electrons in an atom can increase at the time of reaction due to the excitation of electron from one orbital to the orbital of slightly higher energy.
6. Number of unpaired electrons in ground state or excited state of an atom is called covalency of the element.
Hybridization
↪ Hybridization may be defined as the phenomenon of intermixing of the orbitals of slightly different energies so as to redistribute their energies and to give new set of orbitals of equivalent energy and shape.
↪ The new orbitals formed as a result of hybridization are called hybrid or hybridized orbitals.
The important characteristics of hybridization are listed below:
↪ The number of hybridized orbitals formed is equal to the number of orbitals that get hybridized.
↪ The hybridized orbitals are always equivalent in energy and shape.
↪ The hybrid orbitals are more effective in forming stable bonds than the pure atomic orbitals.
↪ The hybrid orbitals are directed in space in some preferred directions to have stable arrangements.
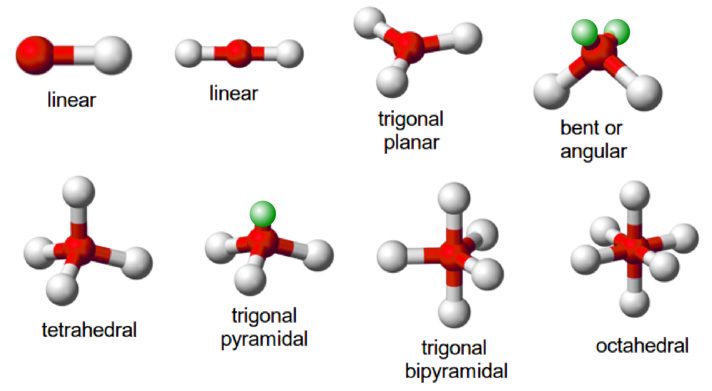
Therefore, the type of hybridization gives the geometry of the molecule. Depending upon the different combinations of s- and three p-orbitals, three types of hybridizations are known.
sp hybridization:
↪ This involves the mixing of one s- and one p-orbital forming two sp-hybrid orbitals.
↪ The two sp-hybrid orbitals are oriented in a linear arrangement and bond angle is 180°.
For e.g., BeF2 involves sp-hybridization
sp2 hybridization:
↪ In this case, one s- and two p-orbitals hybridize to form three sp2 hybrid orbitals.
↪ These three sp2 hybrid orbitals are oriented in a trigonal planar arrangement.
For e.g., in BH3 boron atom undergoes sp2 hybridization and therefore, BH3 has trigonal planar geometry and HBH bond angle is 120°. 
sp3 hybridization:
↪ In this case, one s- and three p-orbitals hybridize to form four sp3 hybrid orbitals.
↪ These four sp3-hybrid orbitals are oriented in a tetrahedral arrangement.
↪ The common example of molecule involving sp3-hybridisation is methane (CH4). Therefore, CH4 has tetrahedral geometry and HCH bond angle is 109.5°