Faraday's Law of Electrolysis
Before moving into Faraday's Law of Electrolysis, let's talk about electrochemistry and some terms related to it. Electrochemistry The branch of chemistry that deals with the study of relation...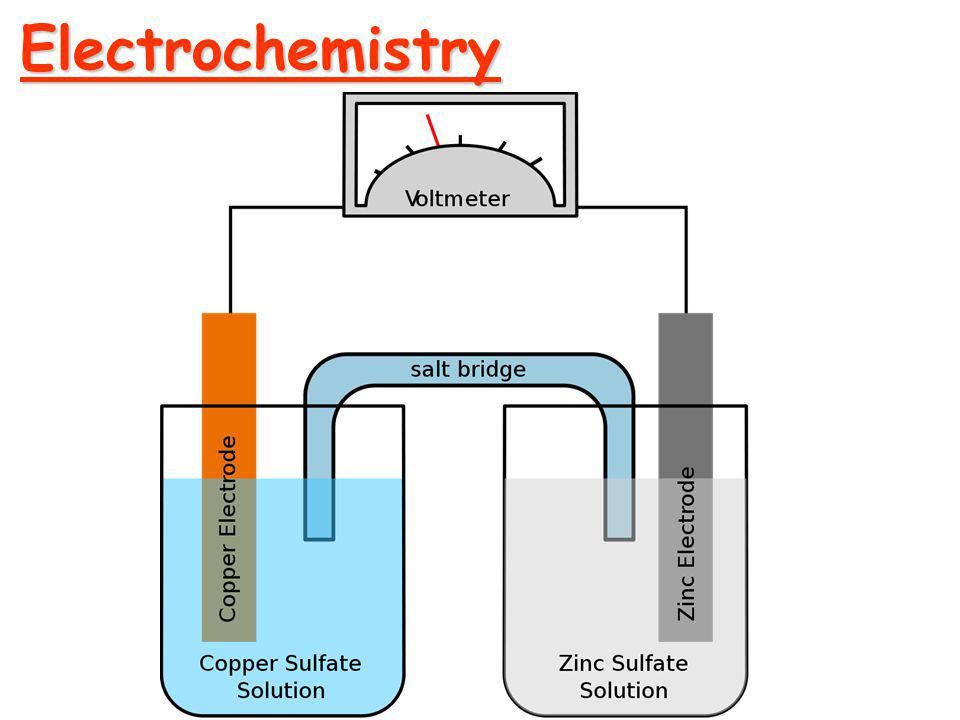

For the verification of second law of electrolysis, the following experimental arrangement is performed:
It consists of various voltameters say copper voltameter, silver voltameter and zinc voltameter. These voltameters have their corresponding electrolytes. The voltameter containing copper sulphate solution as the electrolyte is called copper voltameter. The voltameter containing silver nitrate solution as the electrolyte is called silver voltameter, whereas the voltameter containing zinc sulphate solution as the electrolyte is called zinc voltameter. These voltameters have their corresponding electrodes. Anode and cathode are respectively connected to positive and negative gm terminals of a cell. The ammeter measures the amount of current and the rheostat controls the current.
Before passing the current through the electrolytes, measure the masses of electrodes. Also note down chemical equivalence of copper sulphate, zinc sulphate and silver nitrate. Now pass the constant current for a certain time and note down the masses of ions liberated or deposited on the electrodes of respective voltameters.
If M1, M2 and M3 are the masses of ions liberated or deposited on the electrodes of copper voltameter, silver voltameter and zinc voltameter respectively and E1, E2 and E3 are the chemical equivalences of copper sulphate, silver nitrate and zinc sulphate respectively then it will be seen as:
M1/E1 = M2/E2 = M3/E3
M/E = constant
M = constant * E
M ∝ E
This proves that mass is proportional to Electrochemical equivalence.