Faraday's Law of Electrolysis
Before moving into Faraday's Law of Electrolysis, let's talk about electrochemistry and some terms related to it. Electrochemistry The branch of chemistry that deals with the study of relation...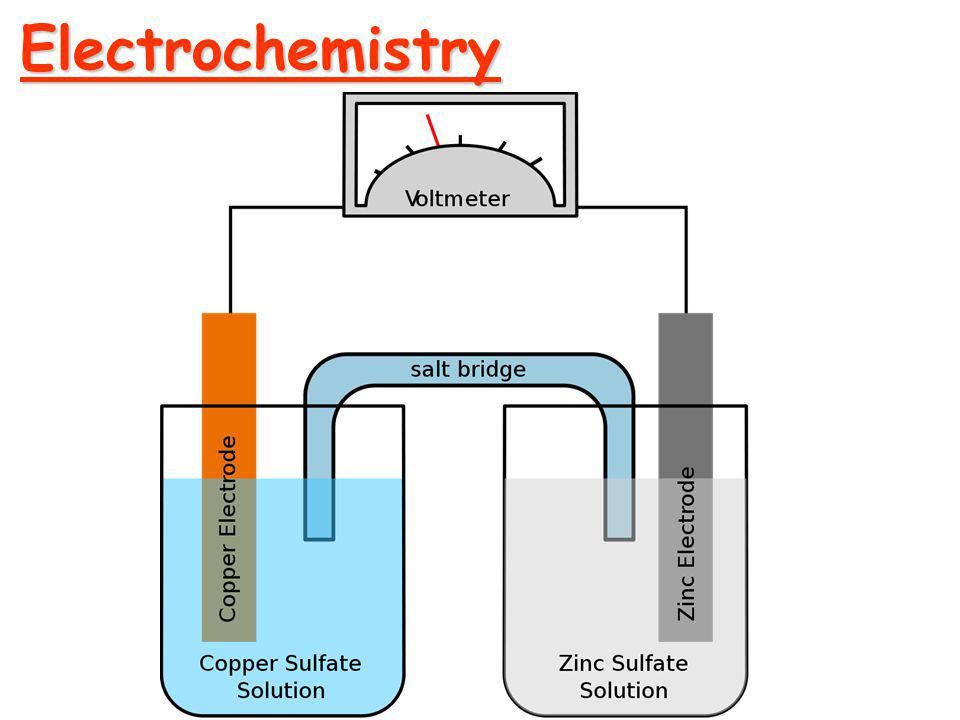

Electrolysis
The process of decomposition of a molecule of compound into it's constituent positive and negative ions, when the current is passed through it is called electrolysis.

The chemical or compound which decomposes is called electrolyte and the vessel in which the experiment is carried out is called a voltameter. There are two metallic plates called electrodes that are immersed in the electrolyte which are respectively connected to positive and negative terminals of a cell. The electrode connected with the positive terminal is called an anode, and the electrode connected with the negative terminal is called a cathode. The positive ions are attracted towards the cathode called cations, and the negative ions are attracted towards anode called anion. So, there is the movement of ions in the electrolyte called ionic conduction.
When no current is passed through the electrolyte, there is strong Coulomb's electrostatic force of attraction between positive ions, and negative ions in the molecule given by:

where, q1 = Charge on positive ion
q2 = Charge on negative ion
r = distance between q1 and q2
But when the current is passed through the electrolyte, the Coulomb's force between the ions decreases and hence positive and negative ions are separated from each other from the molecule and move through the electrolyte, which is ionic conduction.
Faraday’s laws of electrolysis:
Faraday formulated two laws of electrolysis:
Faraday’s first law:
It states that “the mass of ions liberated or deposited on the electrodes is directly proportional to the amount of charge passing through the electrolyte”.
If m is the mass of ions liberated or deposited on passing charge q then;
m ∝ q
m = Z. q,
where Z is a constant and is called electrochemical equivalence of the electrolyte.
If q = 1 then Z = m ,
Hence, electrochemical equivalence is defined as the mass of ions liberated or deposited on the electrodes when the unit charge is passed through the electrolyte.
Units of Z :-
Since Z = m / q
In S.I system, Z = kilogram /coulomb = kg/C
Faraday’s second law of Electrolysis:
It states that “the masses of ions liberated or deposited on the electrodes in various electrolytes are proportional to their chemical equivalence.”

If M1, M2 and M3 are the masses of ions liberated or deposited on the electrodes of sulphuric voltameter, copper voltameter and silver voltameter respectively and E1, E2 and E3 are the chemical equivalences of sulphuric acid, copper sulphate and silver nitrate respectively then it will be seen as:
M1/E1 = M2/E2 = M3/E3
M/E = constant
M = constant * E
M ∝ E
This proves that mass is proportional to Electrochemical equivalence.