Write the truth table of the OR gate and the NAND gate. How the OR gate is drawn by using the NAND gate?
Considering, 1 as high input/output and 0 as low input/output
OR Gate (Truth Table)
| First Input(A) | Second Input(B) | Output(Y) |
| 1 | 1 | 1 |
| 1 | 0 | 1 |
| 0 | 1 | 1 |
| 0 | 0 | 0 |
NAND Gate (Truth Table)
| First Input(A) | Second Input(B) | A.B(AND output) | NAND Output(Y) |
| 1 | 1 | 1 | 0 |
| 0 | 1 | 0 | 1 |
| 0 | 0 | 0 | 1 |
| 1 | 0 | 0 | 1 |
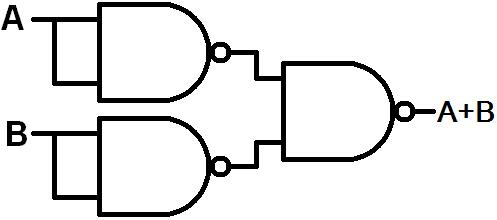
As shown in the above figure, three NAND gates are...

