Introduction to Semiconductors


J.J Thomson clearly identified electron as elementary particle in 1897. Electron is the basic component of all matter. It has a negative charge of 1.602 * 10-19 C and a mass of 9.1085 *
10-31 kg at rest.
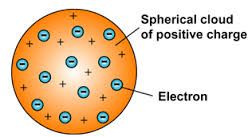
The postulates of Thomson's atomic model are:
1. The atom is a positively charged sphere with electrons distributed in it.
2. The total negative charge of the electron is equal to the total positive charge in an atom.
Failure of Thomson's Atomic Model
1. It could not explain the origin of spectral series ( line spectrum ) as in case of hydrogen atom.
2. It could not explain the large angle scattering of α - particles from thin metal foils as
observed by Rutherford.