Nuclear binding energy
Nuclear binding energy is the minimum energy that would be required to disassemble the nucleus of an atom into its component parts. These component parts are...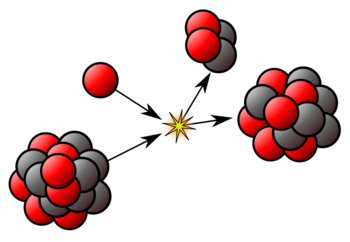

Nuclear Fission
When a heavy nucleus like 92U235 is bombarded by a neutron, the total mass of nuclei is not equal to the sum of the masses of the heavy nucleus and the neutron. Similarly, when two light nuclei like 1H2 fused together to form a heavier and stable nucleus, the mass of the product is not equal to the sum of masses of the initial lighter nuclei. This shows that there is always difference in mass, called mass defect, in a nuclear reaction. The difference in mass is converted into energy which is known as nuclear energy.
Nuclear Fission
When a heavy unstable nucleus of an atom breaks up into two or more lighter nuclei, a large amount of energy is released. This phenomenon is called the nuclear fission. For example,
0n1 + 92U235→ [92U235] → 56Ba141 + 36Kr92 + 30n1 + Energy
Energy released in Fission
The energy is released in the process due to the difference in mass of initial nucleus and its products. The initial mass is greater than the sum of masses of the products and the difference in mass(Δm) is converted into energy according to Einstein's mass energy relation E = Δmc2.
Chain Reaction
A chain reaction is a self propagating process in which number of neutrons goes on multiplying rapidly almost in geometrical progression during fission till all the fissionable material is disintegrated. There are two types of chain reaction.
Multiplication Factor
The ratio of secondary neutrons produced to the initial number of neutrons is called multiplication factor. It is denoted by k.
k = 
If k = 1, the reaction is steady or critical
If k > 1, the reaction is building up or super-critical
If k < 1, the reaction is dying down or sub-critical
Critical Size for Maintenance of Chain Reaction
The critical size of a system containing fissile material is defined as the minimum size for which the number of neutrons produced in the fission process just balance those lost by leakage and non fission capture.
Nuclear fusion
Nuclear fusion is a process in which two or more lighter nuclei combine or fuse together to form a heavier and stable nuclide. In nuclear fusion, the sum of mass of lighter nuclei is always greater than the mass of heavy nucleus. The difference in mass (Δm) resulting in the process is converted into energy according to Einstein's mass energy relation E = Δmc2 which is released during fusion reaction. For example,
1H2 + 1H2 →2He4 + Energy
The Fusion Reaction
Fusion reactions constitute the fundamental energy source of stars, including the Sun. Hydrogen "burning" initiates the fusion energy source of stars and leads to the formation of helium. Generation of fusion energy for practical use also relies on fusion reactions between the lightest elements that burn to form helium.
Reaction Energy
The difference between the masses before and after the reaction corresponds to the reaction energy, according to the mass-energy relation E = Δmc2. If initial particles A and B interact to produce final particles C and D, the reaction energy Q is defined as
Q = (MA + MB - MC - MD) c2