Nuclear binding energy
Nuclear binding energy is the minimum energy that would be required to disassemble the nucleus of an atom into its component parts. These component parts are...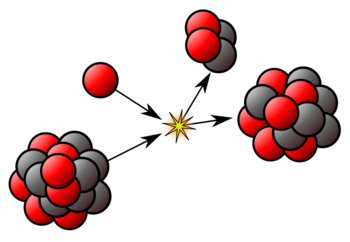
Atomic Mass and Atomic Mass Unit
Mass refers to the mass of a neutral atom. Thus, this includes the masses of corresponding nucleons, the masses of the orbital electrons and the mass equivalent to their binding energy. Being too small, the masses of atoms, nucleus and fundamental particles (electrons, protons neutrons etc.) are expressed in a unit called atomic mass unit, abbreviated as amu or u. 1 amu is defined as 1/12 of the mass of 6C12 atom. As 12 g of 6C12 contains 6.023 X 1023 atoms of 6C12 ,so mass of 6C12 atom is
12/(6.023 X 1023 )g
By definition,
1 amu = 1/12 (12/(6.023 X 1023)g
=1/(6.023 X 1023)g
=1.663 X 1024 g
1 amu = 1.66 X 10-27 kg
On atomic scale, mass of protons, neutrons and electrons are,
m p = 1.672622*10-27 kg = 1.007276u
m n = 1.674927*10-27 kg = 1.008665u
me = 9.10938* 10-31kg = 0.000548580u
Atomic Mass Unit in Terms of Energy
According to Einstein's mass energy relation E = mc2, the energy equivalent to 1 amu ( 1.66 X 10-27) is given by,
E = 1.66 X 10-27X (3 X 108)2 J = 1.49 X 10-10 J
But 1.6 X 10-19 J = 1 eV, so
E = ( 1.49 X 1010)/(1.6 X 10-19 ) eV
= 0.931 X 10 9 eV
= 931 MeV
\ Energy equivalent of 1 amu = 931 MeV
It can be proved that the energy equivalent of the mass of an electron, proton and neutron are respectively given by
me = 0.511 MeV
m p = 938.279MeV
m n = 939.573 MeV
Types of Nuclei
1.Isotopes
Isotopes are the nuclei having the same atomic number Z but different mass number A. Isotopes of an element have identical chemical behavior ( due to same atomic number which determine chemical properties) and differ physically only in mass.
Isotopes of some elements are:
1. Hydrogen : 1H1, 1H2, 1H3
2. Helium : 2He3, 2He4
3.Carbon : 6C14, 6C12
2.Isobars
Isobars are the nuclei having the same mass number but different atomic number. These are the nuclei of different elements having different physical and chemical properties. Some of the examples of isobars are :
1. 1H3 and 2He3
2. 7N14 and 6C14
3.20Ca40 and 18Au40
3. Isotones
Isotones are the nuclei having the same neutron number. Some examples of isotones are:
1. 2He4 and 1H3 (N = 2)
2. 1H2 and 2He3 (N = 1)
3.7N17, 8O18 and 9F19( N = 10)