Chemical Bonding and Shape of Molecules

↪ Valence Shell Electron Pair Repulsion (VSEPR) theory is a simple theory to predict and explain the shape and bond angles in a number of simple covalent molecules and ions.
↪ This theory was proposed by Sedgwick and Powell in 1940 initially and developed by Gillespie and Nyholm in 1957 as VSEPR theory.
The main postulates of this theory may be summarized as follows;
↪ The shape of the molecule is determined by both the total number of electron pairs (bonding and non-bonding) around the molecules central atom and the orientation of these electron pairs in the space around the central atom.
↪ In order to minimize the repulsion forces between them, electron pairs around the molecules central atom, tend to stay as far away from each other as possible
↪ Electron pairs around the molecule's central atom can be shared or can be lone pairs. The 'shared pairs' of electrons are also called bond pairs of electrons. The presence of lone pair(s) of electrons on the central atom causes some distortions in the expected regular shape of the molecule.
↪ The strength of repulsions between different electron pairs follows the order:
Lone pair - Lone pair > Lone pair - Shared pair > Shared pair - Shared pair.
Prediction of molecular geometry on the basis of VSEPR theory:
Molecule with two bond pairs:
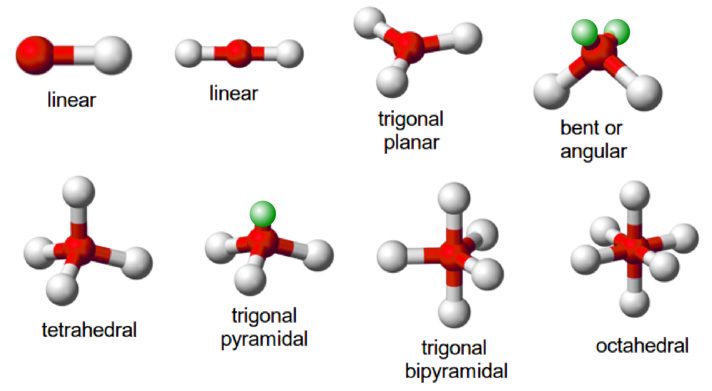
↪ In a molecule having two bond pairs of electrons around its central atom, the bond pairs are located on the opposite sides (at an angle of 180o) so that the repulsion between them is minimum.
↪ Such molecules are therefore linear. Some molecules, which show linear geometry are: BeF2 (beryllium fluoride), BeCl2(beryllium chloride), BeH2 (beryllium hydride), ZnCl2 (zinc chloride), and HgCl2(mercuric chloride)

↪ In a molecule having three bond pairs of electrons around its central atom, the electron pairs form an equilateral triangular arrangement around the central atom. These molecules have trigonal planar (or triangular planar) shape and the three bond pairs are at 120°C with respect of each other. Some molecules that show triangular planar geometry are BCl3, BF3, etc.

boron trifluoride is a trigonal planar molecule

