1. Radioactive Decay:
- Definition: Radioactive decay is a spontaneous process in which an unstable atomic nucleus undergoes a change, resulting in the emission of radiation.
- Mechanism: It occurs when an atomic nucleus is unstable due to an imbalance between the number of protons and neutrons in the nucleus. The unstable nucleus transforms itself into a more stable configuration by emitting radiation.
- Energy Release: In radioactive decay, energy is released in the form of various types of radiation, such as alpha particles, beta particles, or gamma rays.
- Chain Reaction: Radioactive decay is not a chain reaction process, as it happens independently in each unstable nucleus.
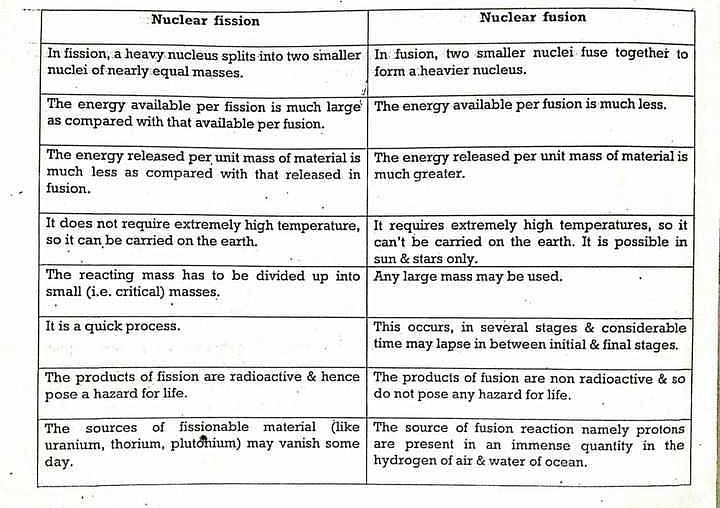
2. Nuclear Fission:
- Definition: Nuclear fission is a process where the nucleus of an atom is split into two or more smaller nuclei, accompanied by the release of a large amount of energy.
- Mechanism: It typically occurs when a heavy atomic nucleus, such as uranium-235 or plutonium-239, absorbs a neutron and becomes highly unstable. The nucleus then splits into two or more smaller nuclei, known as fission fragments, and additional neutrons are released.
- Energy Release: Nuclear fission releases an enormous amount of energy in the form of kinetic energy of the fission fragments and additional neutrons. This energy is typically harnessed for various applications, such as electricity generation in nuclear power plants.
- Chain Reaction: Nuclear fission can sustain a chain reaction under certain conditions. When the released neutrons go on to induce fission in other nuclei, and those fission reactions release more neutrons, a self-sustaining chain reaction occurs. This chain reaction can be controlled in nuclear reactors or can be uncontrolled in nuclear weapons.