Potentiometer
The potentiometer is an accurate device that is used to measure the emf or potential difference more accurately. It is accurate than a voltmeter in the sense that the voltmeter used for measuring p...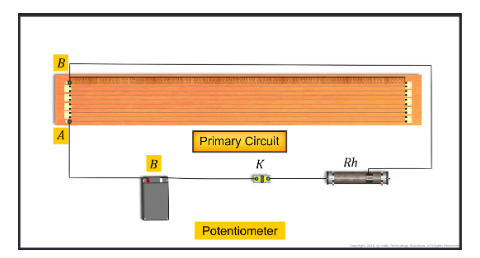

When two terminals of a battery are connected by a conductor, an electric current flows through the conductor. One terminal continuously sends electrons into the conductor, while the other continuously receives electrons from it. In this way, the battery behaves like an electric pump. The property of a cell (or generator) that makes the charge move in a particular direction is called electromotive force or emf. Just like a force that moves a mass, an emf moves a charge in a circuit. The emf of a source is not a force but work done in moving a unit charge around the circuit
The emf of a cell is the energy supplied by the cell to move unit charge around a circuit join to it. It the potential difference between the terminals of the cell in an open circuit. It does not depend on the size of a cell but it depends on the chemical used in it. It is denoted by E. Its unit is volt in SI-unit
Internal Resistance
The resistance offered by a source for a current to pass through it is called its internal resistance. The internal resistance of a cell is the resistance offered by the electrolyte between the electrode of the cell when an electric current passes through it Internal resistance of a fresh new cell is small but the resistance increases as we use it more and more. It is shown externally in series to the cell as shown in
Fig It is denoted by r
Figure Representation of internal resistance
The internal resistance of a cell depends on many factors
The distance between the electrodes: r increases with an increase in distance between the
electrodes.
The nature of the electrolyte, greater the conductivity of the electrolyte, lesser is the internal resistance
Nature of the electrodes
The area of the plates or electrodes immersed in the electrolyte: internal resistance decreases with an increase in the area of the plates immersed
The temperature of the electrolyte, internal resistance decreases with the rising of temperature.