Transfer of Heat
INTRODUCTIONSimply heat is the sum of kinetic energy of atoms or molecules. In thermodynamics, heat means energy which is moved between two things when one of them is hotter than the other.Heat ene...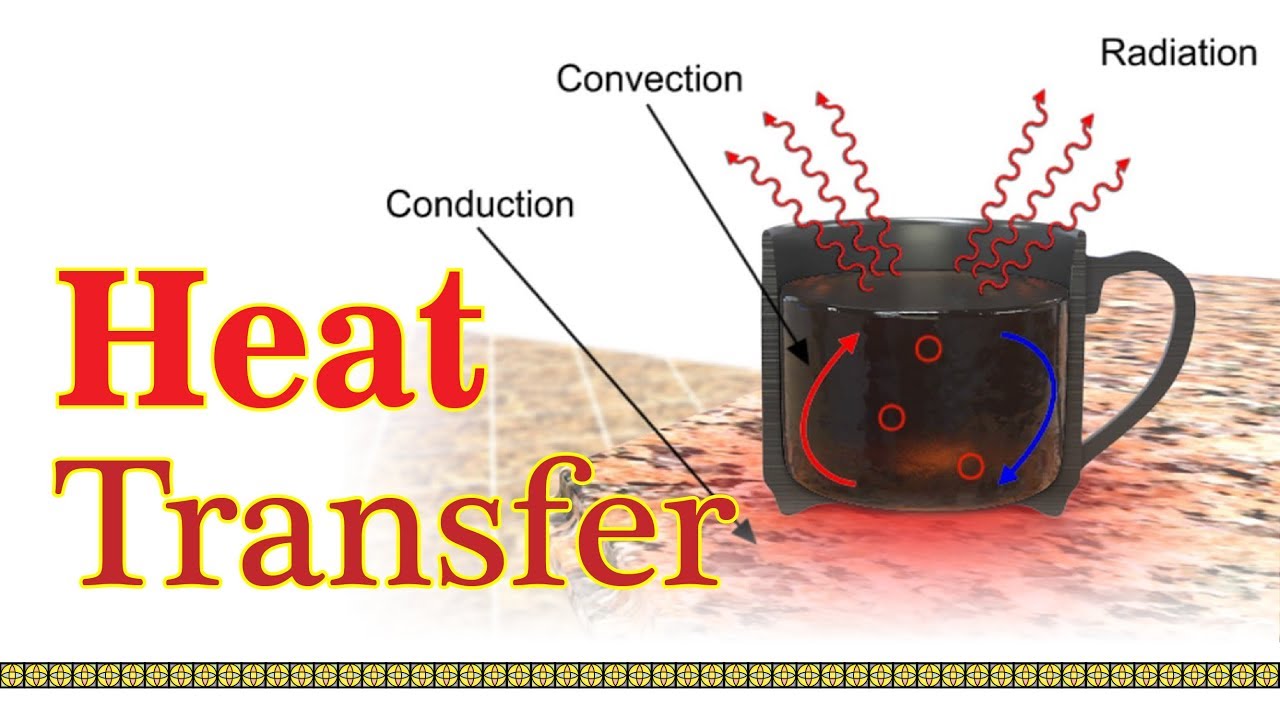

Heat Pumps and Refrigerators
In a heat engine, the direction of energy transfer is from the hot
reservoir to the cold reservoir, which is the natural direction. The role of the heat
the engine is to process the energy from the hot reservoir so as to do useful work. What if we wanted to transfer energy by heat from the cold reservoir to the hot reservoir?
Because this direction is not the natural one, we must transfer some energy into a device to cause it to occur.
Devices that perform this task are called heat pumps or refrigerators.

Active Figure 18.7 is a schematic representation of a heat pump.
The cold reservoir temperature is Tc , the hot reservoir temperature is Th and the energy
absorbed by the heat pump is |Q c |. Energy is transferred into the system,
which we model as work W, and the energy transferred out of the pump is |Q h |. Heat pumps have long been popular for cooling in homes, where they are called air conditioners and are now becoming increasingly popular for heating purposes as well. In the heating mode, a circulating coolant fluid absorbs energy from the outside air (the cold reservoir) and releases energy to the interior of the structure (the hot reservoir). The fluid is usually in the form of a low-pressure vapor when in the coils of the exterior part of the unit, where it absorbs energy from either the air or the ground by heat. This gas is then compressed into a hot, high-pressure vapor and enters the interior part of the unit, where it condenses to a liquid and releases its stored energy. An air conditioner is simply a heat pump installed backward, with “exterior” and “interior” interchanged. The inside of the home is the cold reservoir and the outside air is the hot reservoir. The effectiveness of a heat pump is described in terms of a number called the coefficient of performance COP. In the heating mode, the COP is defined as the ratio of the energy transferred by heat into the hot reservoir to the work required to transfer that energy:

As a practical example, if the outside temperature is 269 K or higher, the COP for a typical heat pump is about 4. That is, the energy transferred into the house is about four times greater than the work done by the compressor in the heat pump. As the outside temperature decreases, however, it becomes more difficult for the heat pump to extract sufficient energy from the air, and the COP therefore drops. A Carnot cycle heat engine operating in reverse constitutes an ideal heat pump, the heat pump with the highest possible COP for the temperatures
between which it operates. The maximum coefficient of performance is

Although heat pumps are relatively new products in heating, the
refrigerator has been a standard appliance in homes for decades. The refrigerator
cools its interior by pumping energy from the food storage compartments into the
warmer air outside. During its operation, a refrigerator removes energy |Q c | from the interior of the refrigerator, and in the process its motor does work W on the coolant fluid. The COP of a refrigerator or of a heat pump used in its cooling cycle is

An efficient refrigerator is one that removes the greatest amount of energy from
the cold reservoir with the least amount of work. Therefore, a good refrigerator should have
a high coefficient of performance, typically 5 or 6. The highest possible COP is again that of a refrigerator whose working the substance is carried through the Carnot heat engine cycle in reverse:

As the difference between the temperatures of the two reservoirs approaches zero,
the theoretical coefficient of performance of a Carnot heat pump approaches infinity. In practice, the low temperature of the cooling coils and the high
the temperature at the compressor limits the COP to values below 10.