A detailed study on Nitrogenous fertilizers.
There are 4 types of nitrogenous fertilizers. They are; Nitrate Fertilizers, Ammonium Fertilizers, Nitrate and Ammonium Fertilizers, and Amide Fertilizers. There are many uses and many harmful effe...

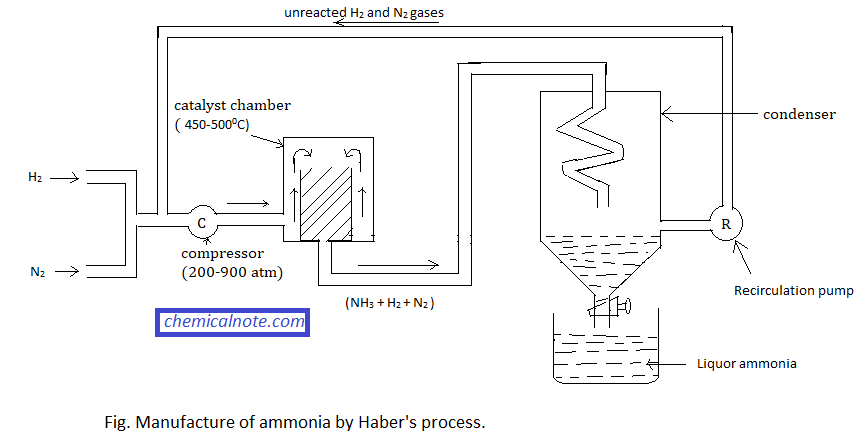
↪ A German professor of chemistry Dr. Fritz Haber (1868 - 1934), invented a new method for manufacturing ammonia gas.
↪ It is a synthetic process known after his name as the Haber's process.
↪ He received the Nobel prize in chemistry in 1918.
↪ Mostly ammonia is prepared now-a-days by this process.
Theory
↪ When a mixture of one volume nitrogen and three volume hydrogen in the dry state in passed under a pressure of 200-900 atmosphere through a chamber packed with catalysts containing of finely divided iron with a little metal oxides like Al2O3, ZrO and K20 as a promotor at a temperature of about 450-500°C, the two gases combine under these conditions to form about 10% of ammonia, according to the reaction.

↪ Since this reaction is gaseous, reversible and exothermic, Le-Chatelier's principle would help to suggest the most favorable conditions for the manufacture of ammonia and it is explained as follows:
⁕ Low optimum temperature
↪ Since the reaction is exothermic, according to Le-Chatelier's principle low temperature will favor the forward reaction.
↪ Though according to the principle low temperature increases the yield of ammonia yet the reaction rate becomes too slow in reaching the equilibrium and so for effective production, Haber compromised the temperature of 450°C in the process.
↪ This temperature is the optimum temperature (or compromised temperature) applied in Haber synthesis.
⁕ High pressure
↪ Formation of ammonia from hydrogen and nitrogen results in decrease of the volume.
↪ Therefore high pressure would favor the formation of ammonia.
1 atm. and 450°C → 0.13%
200 atms. and 450°C → 17.6%
1000 atms. and 450°C → 40%
↪ Data showed that the yield of ammonia increases with the increase of pressure.
⁕ High concentration
↪ If excess of either hydrogen or nitrogen is taken then, again, the concentration of ammonia increases because the principle states that equilibrium shifts to forward direction as the concentration of reactant at equilibrium is increased.
⁕ Catalyst
↪ It may be noted that the catalyst does not increase the amount of the product but it brings the equilibrium state in a short time.
↪ Reaction is slow in the absence of catalyst.
↪ Finely divided iron containing metal oxides like Al2O3, ZrO and K2O as a promoter, has been used as a catalyst for the rapidity of reaction. A mixture of Fe3O4 and K,O.Al2O3 (admixture) has also been used.
↪ This mixture makes Fe catalyst active throughout the process.
⁕ Purity of H2 and N2
↪ The mixture of hydrogen and nitrogen should be very pure, otherwise the catalyst is poisoned.
↪ This decreases the efficiency of the catalyst.