↪ In general, there are three major metallurgical operations for the extraction of a metal from its ore.
↪ Those are:
⇲ Ore dressing and concentration
⇲ Extraction of metal from concentrated ore
⇲ Purification of metal
Depending upon the nature of ore, metal and impurities, general principles involved behind each of the operations are described below:
Ore dressing and concentration
⁕ Crushing and pulverization
↪ The collected big lumps of ore are crushed in jaw crushers and then pulverized in ball mills or stamp mills.
↪ The powdered ore is then taken for concentration.
⁕ Concentration
↪ The process of removal of impurities like sand, rocky materials, earthy particles etc. are removed from powdered ore is known as concentration.
↪ In this operation, percentage of the metal in the ore is increased.
↪ Hence it is called concentration process.
↪ Concentration is carried out by any one of the following methods:
⇲ Hand picking
↪ The process of picking up impurities from ores by hand is termed as hand picking.
↪ It is done when the impurities are quite distinct and large in the size.
⇲ Gravity separation (Levigating)
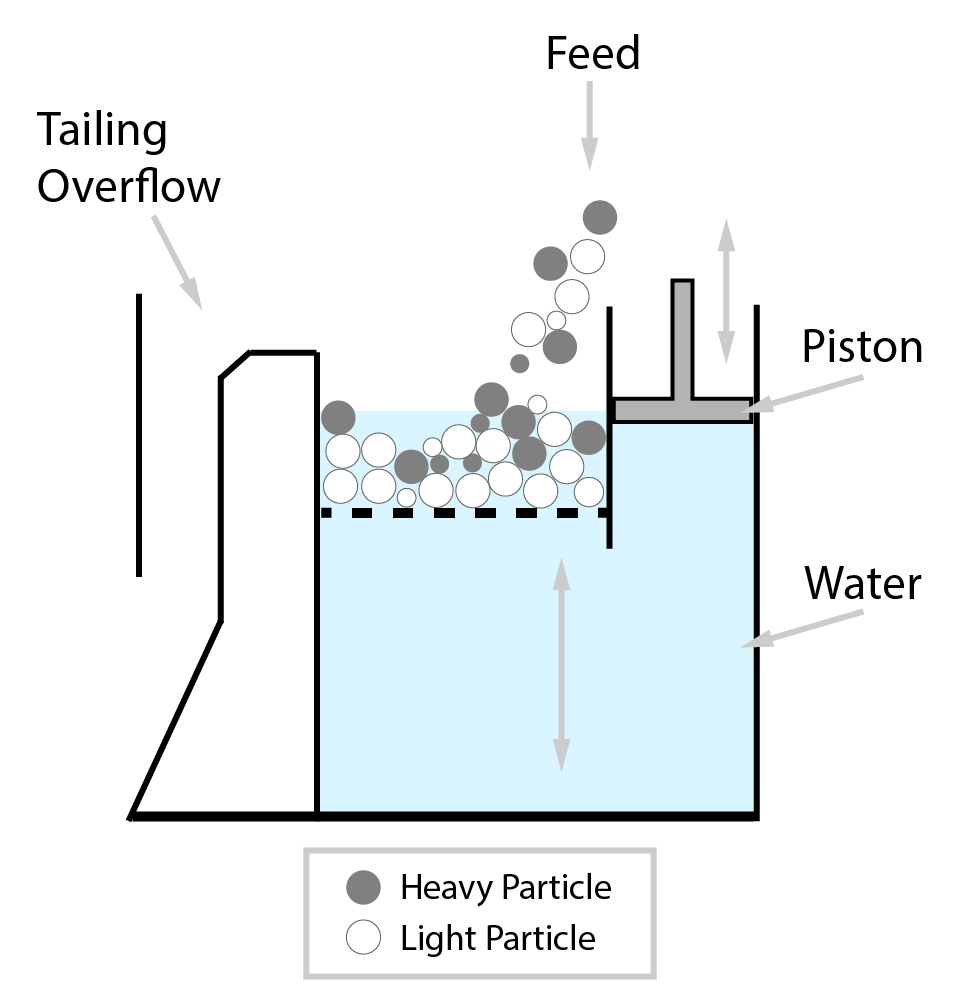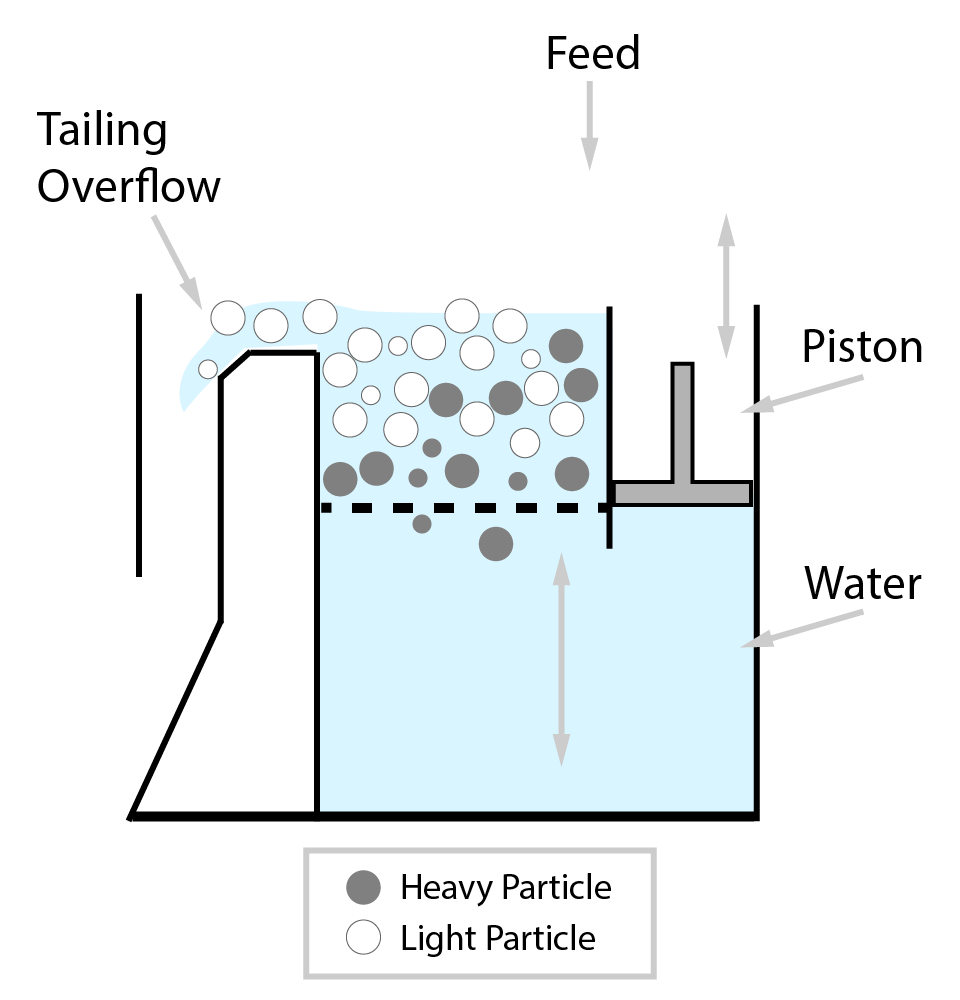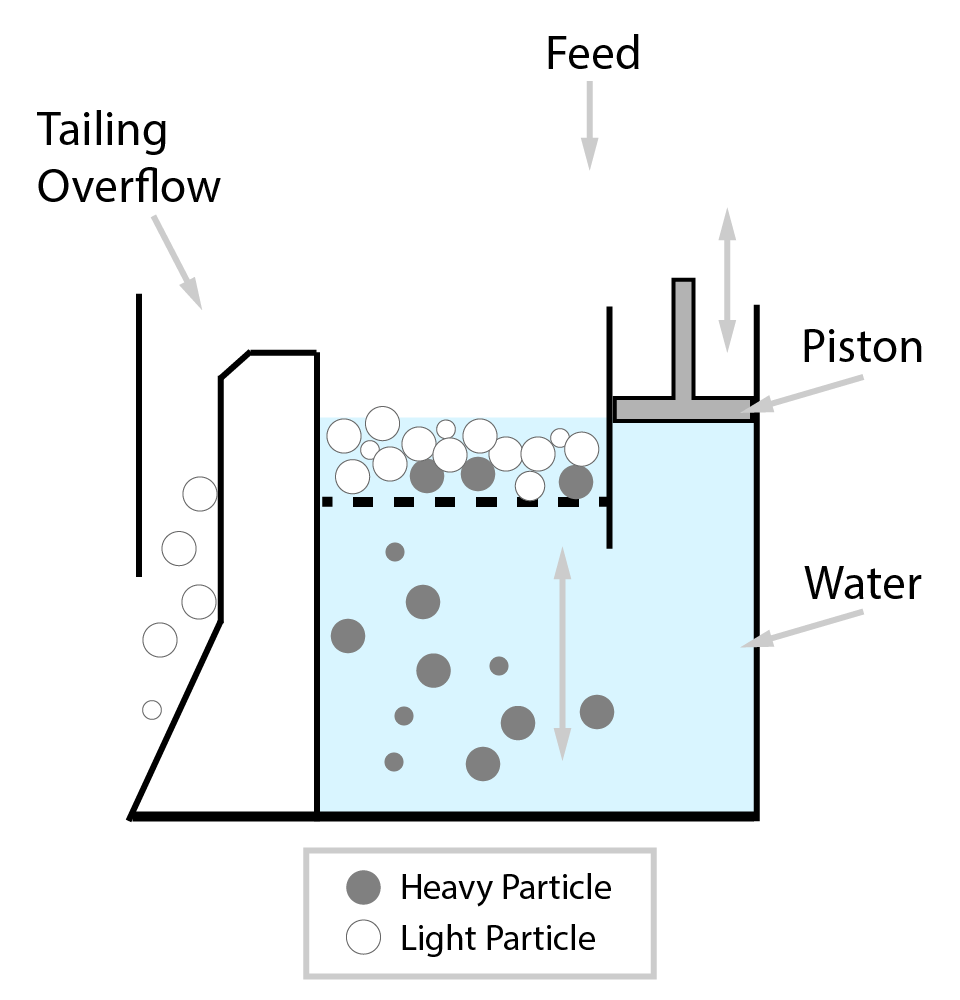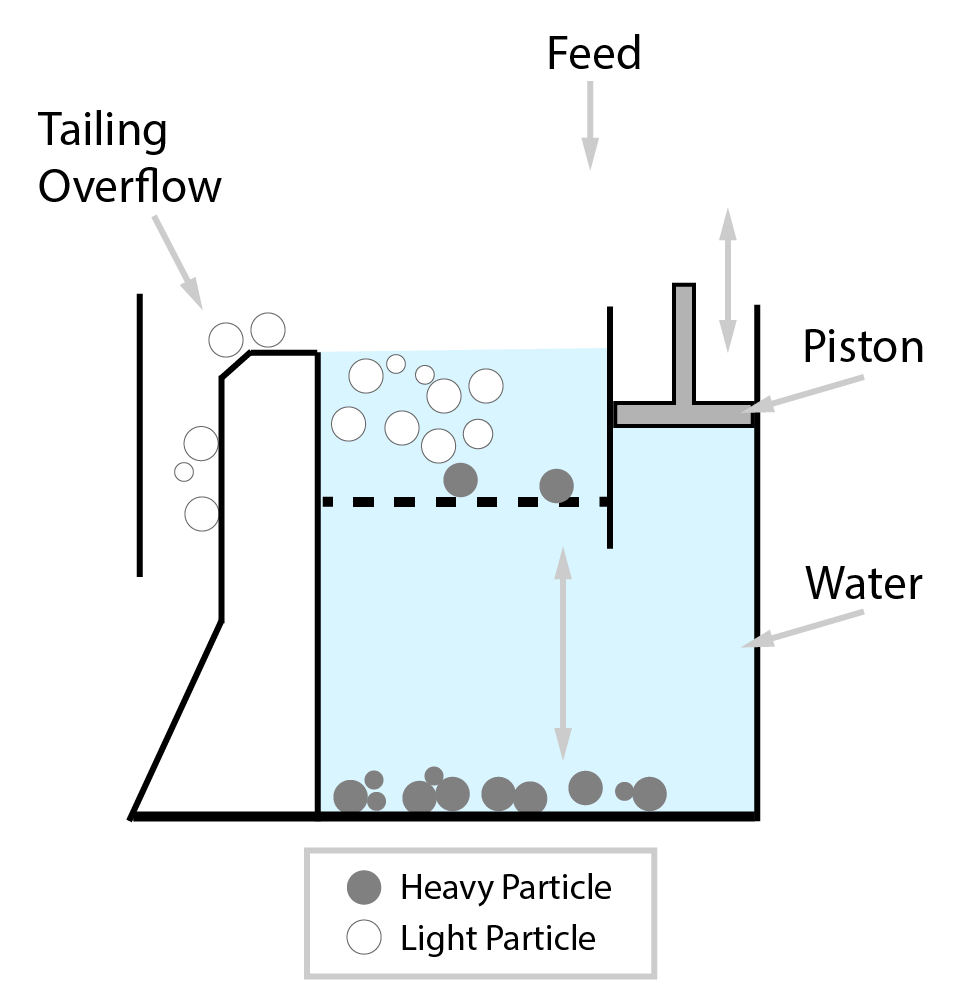
↪ The process of separation of impurities (gangue particles) from the ore due to their different specific gravity is known as gravity separation.
↪ In this process, the lighter gangue particles are washed away by upward stream of running water while heavier ore particles settle down and removed from the base.
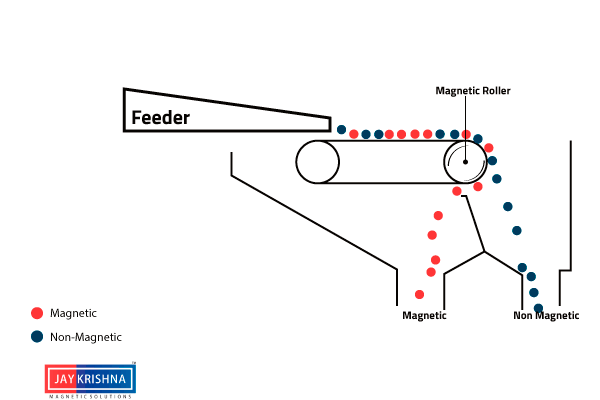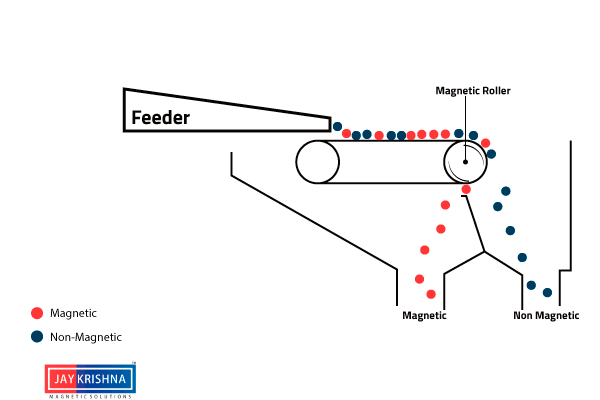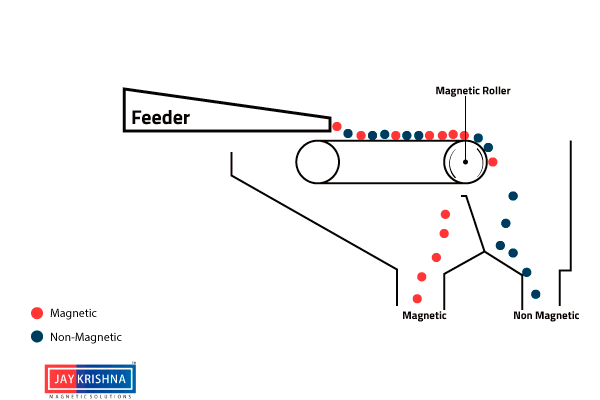
⇲ Magnetic separation
↪ This method is based on the difference in the magnetic properties of the ore and impurities.
↪ Magnetic Ore having non magnetic impurities or non magnetic ore having magnetic impurities can be concentrated by this method.
↪ The pulverized ore is dropped on a travelling belt passing over magnetic wheel where magnetic particles are pulled toward magnetic wheel and non magnetic particles get deposited in separate heap.
⇲ Froth floatation process
↪ This process is commonly employed for the concentration of sulphide ores like zinc blende (Zns), Galena (PbS),Cinnabar (HgS).
↪ In this process, the ore is mixed with water containing a little pine oil in a big tank and agitated by blowing blast of air.
↪ The ore particles come to surface along with froth and are skimmed off while gangue particles settle down at bottom.

⇲ Leaching Process
↪ This is the chemical method in which ore is treated with a suitable chemical reagent which selectively reacts with desired metallic compound of the ore leaving behind impurities.
↪ Then the metallic compound is separated from impurities by filtration process.
↪ For example,
When bauxite ore is treated with conc. NaOH solution, aluminum oxide is dissolved leaving behind insoluble impurities, which are then removed by filtration













