Variation of Conductance with Dilution
1. Variation of Specific Conductance.Specific Conductance “К” is the conductance produced by the ions of 1cc of the electrolytic solution. When it is diluted, the concentration of the ions wit...

Ionic Equilibrium
The equilibrium established between the unionized molecules and the ions in the solution of weak electrolytes is called ionic equilibrium.
e.g., CH3 COOH ⇔ CH3COO– + H–
Degree of Ionisation or Degree of Dissociation (α)
It is the fraction of the total number of molecules which ionize (dissociate)into constituent ions. α = (number of molecules ionized or dissociated/total number of molecules taken) For strong electrolytes, α = 1 For weak electrolytes, α < 1
Values of the degree of dissociation depend upon the following factors
Ostwald’s Dilution Law
According to Ostwald. the degree of dissociation of weak electrolytes is inversely proportional to the square root of the molar concentration of the solution.
Here K is the dissociation constant and C is the molar concentration of the solution.
Arrhenius Concept of Acids and Bases
Bronsted Concept of Acids and Bases
Acid is a chemical substance that can donate a proton (H+) to some other substance and a base is a chemical substance that can accept a proton from another substance. Thus, an acid is a proton donor and a base is a proton acceptor.
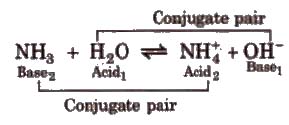
A strong acid has a weak conjugate base and weak acid has a strong conjugate base. A strong base has weak conjugate acid and a weak base has strong conjugate acid.
HClO4 is the strongest while HCN is the weakest hydracid known.
CsOH is the strongest base known.
The amphoteric substance is the substance that acts as an acid as well as a base, e.g.• water acts as an acid with NH3 and a base with acetic acid.
The order of acidic strength of some acids is
HClO4 > HBr> H2SO4 > HC1> HNO3
Greater the Ka value of an acid, the stronger is the acid. Similarly. The greater the Kb of a base, the stronger is the base.
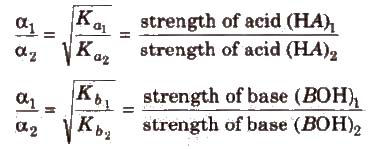
Lewis concept of acid and bases
Lewis proposed broader and more general definitions of acids and bases which do not require the presence of protons to explain the acid-base behaviour. According to Lewis concept. An acid is a substance that can accept a pair of electrons.
Acid-base reactions according to this concept involve the donation of electron pair by a base to an acid to form a co-ordinate bond.
Lewis acids are the species having vacant orbitals in the valence shell of one of its atoms. The following species can act as Lewis acids.
a) Molecules having an atom with incomplete octet e.g., BF3, AlCl3 etc.
b) Simple cations for e.g., H+, Ag+ etc.
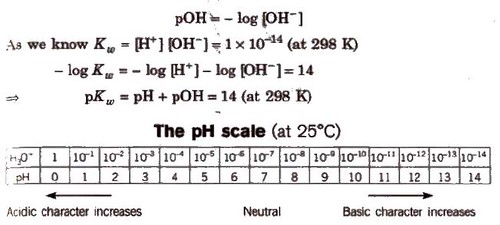
pH and pH scale
pH is the measurement of hydrogen ion concentration. The acidity and alkalinity can be expressed in terms of pH.
The negative logarithm of hydrogen ion concentration expressed in molarity is called pH.
i.e. pH = -log[H+]
Hydrolysis of salts
The process of dissolving salt in water to make its aqueous solution which may be acidic, basic or neutral is called hydrolysis of salt.
It is divided into four categories they are:
a.Salts of strong acid and strong base:
Here, let us consider NaCl as an example. It is a strong electrolyte, when dissolved in water; it goes complete dissociation to give Na+ and Cl- ions. But these ions do not have a tendency to react with H+ or OH-. So, there is no change in concentration of H+ or OH- ions and hence the solution remains neutral.
b.Salts of weak acid and strong base:
Sodium acetate, CH3COONa, NaCN, KCN are examples of this type of salts. Here let us consider sodium cyanide (NaCN) it is the salt of a weak acid, HCN and NaOH. It ionizes to form CN- anions. Being the conjugate base of a weak acid. CN- is a relatively strong base. Thus, the anion CN- accepts an H+ ion from water and undergoes hydrolysis. So, the solution becomes basic due to the generation of OH- ions.
c. Salts of weak bases and strong acids:
Some salts of weak bases and strong acids undergo cationic hydrolysis and yield a slightly acidic solution.
Ammonium chloride is a typical e.g. of these salts. It is the salt of a weak base NH4OH and strong acid HCl. It ionizes in an aqueous solution to form the cation NH4+.
Here, NH4OH+ is a relatively strong acid. It accepts OH- ion from water (H2O) and forms the unionized NH4OH and H+ ion.
So, the accumulation of H+ ions in the solution makes it acidic.
d. Salts of weak acids and weak bases:
The e.g. of this type of salts are ammonium acetate, ammonium cyanide etc. The resulting solution is neutral, basic or acidic depending on the relative hydrolysis of the anions and the cations.
Solubility product principle and its application
When a sparingly soluble ionic solid is dissolved in water it undergoes ionization and the ions forming from the solid phase pass into the solution till the solution becomes saturated and a dynamic equilibrium is established between the ions present in the saturated solution and the ions present in the solid phase at a constant temperature.
Consider a sparingly soluble substance AB which ionized as A+B-
At equilibrium,K = [A+][B−][AB]
Where Ksp = solubility product. The solubility is the product of the concentration of ions of sparingly soluble salts in its saturated solution to a particular temperature. It is represented as Ksp.
The value of Ksp changes with the change in temperature. The value of Ksp gives the idea of the precipitation of an electrolyte at a given temperature.
To precipitate in a chemical reaction, the ionic product must exceed the solubility product of sparingly soluble salt at a particular temperature.
For precipitation.
IP < Ksp; Unsaturated; No ppt.
IP = Ksp; saturated; No. ppt.
IP >Ksp; Supersaturated; ppt.
The solubility product principle is very applicable in analytical chemistry. In salt analysis i.e. (qualitative analysis) the solubility product principle is more applicable. The group separation of metal ions in the salt analysis is done on the basis of the solubility product principle.
Qualitative analysis of solubility product
The common ion effect is generally employed in qualitative analysis.
The cations of group II (Hg2+, Pb2+, Bi3+, Cu2+, As3+, Sb3+, Sn2+) are precipitated as their sulphides (such as CuS, PbS) by passing H2S gas in the presence of hydrochloric acid (Common H+ ions).
The cations of group III are precipitated as their hydroxides by NH4OH in the presence of NH4Cl.
The cations of group V are precipitated as their carbonates by the addition (NH4)2CO3, in the presence of HCl.
Qualitative analysis of solubility product
The common ion effect is generally employed in qualitative analysis.
The cations of group II (Hg2+, Pb2+, Bi3+, Cu2+, As3+, Sb3+, Sn2+) are precipitated as their sulphides (such as CuS, PbS) by passing H2S gas in the presence of hydrochloric acid (Common H+ ions).
The cations of group III are precipitated as their hydroxides by NH4OH in the presence of NH4Cl.
The cations of group V are precipitated as their carbonates by the addition (NH4)2CO3, in the presence of HCl.
Common ion effect and its application
When a soluble salt, say, A+C- is added to a solution of another salt (A+B-) containing a common ion (A+), the dissociation of AB is suppressed
AB⇔A+ + B-
By the addition of the salt, AC, the concentration of A+ increases. Therefore, according to Le Chatelier's principle, the equilibrium will shift to the left, thereby decreasing the concentration of A+ ions. Of that, the degree of dissociation of AB will be reduced.
a salt of a weak acid is added to a solution of the acid itself, the dissociation of the acid is diminished further. For example, the addition of sodium acetate to a solution of acetic acid suppresses the dissociation of acetic acid which is already very small.
Consider the equilibrium,
CH3COOH ⇔ H+ + CH3COO-
The addition of one of the products of dissociation (example: acetate ion), supplied by the largely dissociated salt (example: sodium acetate) pushes the equilibrium to the left. In other words, the dissociation of acetic acid is suppressed. Similarly, the addition of hydrogen ions furnished by the addition of a largely dissociated acid such as hydrochloric acid also suppresses the dissociation of acetic acid.
Thus, the common ion effect is the "suppression of the dissociation of a weak acid or a weak base on the addition of its own ions.
Applications:
A solution that resists any change of pH when a small amount of a strong acid or a strong base is added to it, is called a buffer solution or simply as a buffer.
Alternatively, a buffer solution may be defined as a solution whose pH value does not change appreciably upon the addition of small amounts of a strong acid, base and/or water from outside.
Thus, buffers have reserve acidity and reserve alkalinity.
Buffer solutions usually consist of a mixture of a weak acid and its salt with a strong base e.g., CH3COOH and CH3COONa, or that of a weak base and its salt with a strong acid e.g., NH4OH and NH4Cl. The solution of any salt of a weak acid and a weak base e.g., ammonium acetate, also shows buffering property.